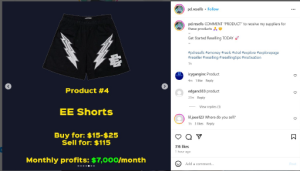
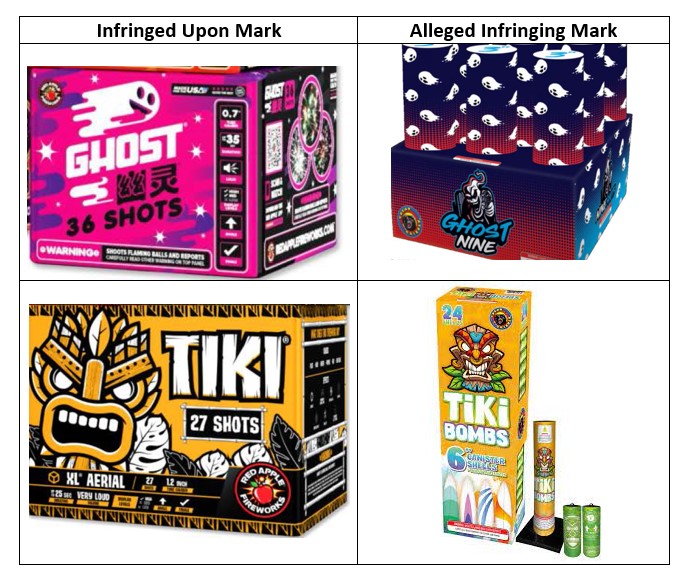
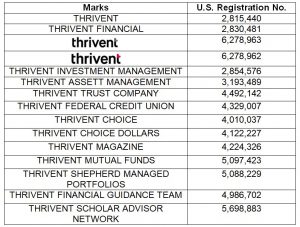
My Healthy Home LLC, a New Jersey-based company focused on promoting healthier living environments, has filed a complaint against the Indiana-based company Healthy Home Experts, LLC, and Joshua Harper for trademark infringement. According to court documents, My Healthy Home has been recognized since 2002 for its environmentally friendly services, including air quality testing and mold remediation, and holds several federally registered trademarks, including HEALTHY HOME EXPERT.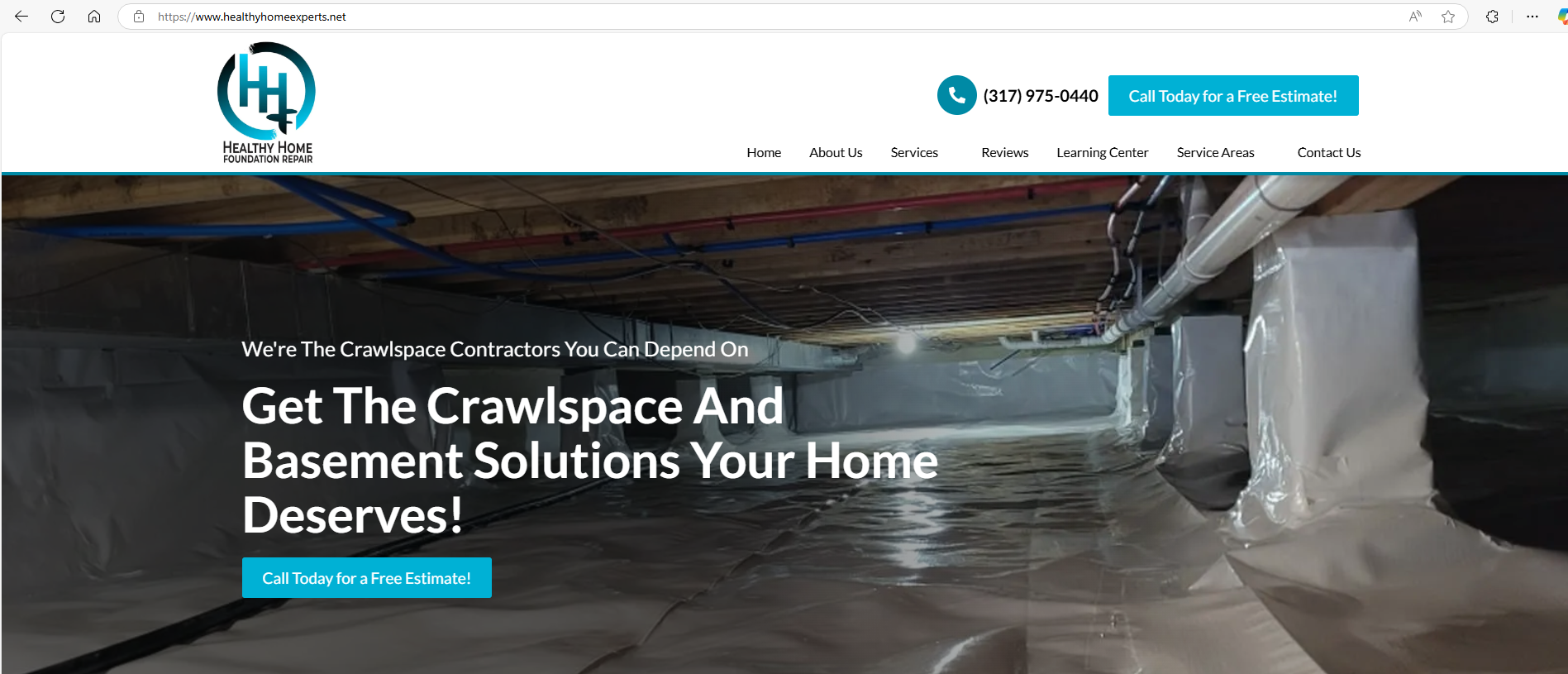
The dispute centers around the defendants registering the domain name https://www.healthyhomeexperts.net/ and forming a business under the name Healthy Home Experts, LLC, which reportedly creates confusion due to its similarity to My Healthy Home’s trademark. The defendants reportedly offer overlapping services, such as air quality testing and mold remediation, using the allegedly infringing name.
My Healthy Home is seeking legal relief for trademark infringement, false designation of origin, cybersquatting, and unfair competition. The company is requesting an injunction to stop the defendants from using the infringing name and domain, as well as damages, including the transfer of the domain to My Healthy Home. They also seek reimbursement for attorney’s fees and compensation for any harm caused to their brand.
 Indiana Intellectual Property Law News
Indiana Intellectual Property Law News