Indianapolis — This week, an attorney from the United States International Trade Commission (USITC) asked Indiana-based Eli Lilly for clarification regarding Lilly’s allegations of patent infringement over the chemotherapy drug gemcitabine. Eli Lilly owns U.S. Patent Number 5,606,048, which is a process of preparing the gemcitabine. The FDA approved gemcitabine for treatment of breast, lung, ovarian, and pancreatic cancer. Lilly alleges that its process is the only commercially viable process of producing the drug and that several companies, including Illinois-based Hospira Incorporated, are making and selling a generic version of the drug using the patented process. In a letter this week, the USITC has asked Lilly to explain why it believes its patented process is being used.
In January, patent attorneys for Eli Lilly filed a complaint with the USITC that alleges patent infringement by unlawful importation and sale of the drug. Lilly seeks an exclusion order from USITC that would ban the importation of the drug into the United States. Lilly alleges that manufactures in China and India use the patented process to make the drug, which Hospira imports and sells in the United States. Patent attorneys for Hospira and Lilly have been litigating over alleged infringement the patent in U.S. and foreign courts for several years. In September, Hospira filed a suit in the United States District Court for Northern Illinois (Case Number 1:2010cv06275) seeking a declaratory judgment stating that Hospira is not infringing Lilly’s patent. According to the Indiana Business Journal, Lilly’s global sales the drug fell 14%, or about $906 million, in the first three quarters of 2010.
Practice Note – When an imported product infringes on a patent, the patent holder can seek an exclusion order from the United States International Trade Commission. USITC patent attorneys will investigate whether the imported product violates U.S. patent law. In prior litigation it appears that Eli Lilly had difficulty proving that its patented process is being used rather than another process. Lilly claims it has been unable to do so because Hospira and the foreign manufacturers have not complied with discovery requests. Nonetheless, it appears from this week’s letter that the USITC is concerned about this factual issue.
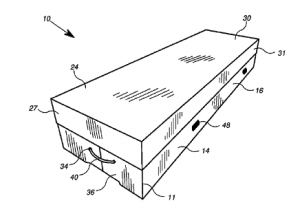 titled “Lightweight Casket Having Foldable Features.” Plaintiff is seeking judgment, preliminary and permanent injunctions, damages, prejudgment and post judgment interest, and attorney’s fees.
titled “Lightweight Casket Having Foldable Features.” Plaintiff is seeking judgment, preliminary and permanent injunctions, damages, prejudgment and post judgment interest, and attorney’s fees. Indiana Intellectual Property Law News
Indiana Intellectual Property Law News


![hdl-test-strips2-new[1].jpg](https://www.iniplaw.org/wp-content/uploads/sites/366/2016/10/hdl-test-strips2-new1.jpg)

 economic treaty, encompassing nations representing more than 40 percent of the world’s gross domestic product (“GDP”). The WikiLeaks release of the text came ahead of the decisive TPP Chief Negotiators summit in Salt Lake City, Utah. The chapter published by WikiLeaks is perhaps the most controversial chapter of the TPP due to its wide-ranging effects on medicines, publishers, internet services, civil liberties and biological patents. Significantly, the released text includes the negotiation positions and disagreements between all 12 prospective member states.
economic treaty, encompassing nations representing more than 40 percent of the world’s gross domestic product (“GDP”). The WikiLeaks release of the text came ahead of the decisive TPP Chief Negotiators summit in Salt Lake City, Utah. The chapter published by WikiLeaks is perhaps the most controversial chapter of the TPP due to its wide-ranging effects on medicines, publishers, internet services, civil liberties and biological patents. Significantly, the released text includes the negotiation positions and disagreements between all 12 prospective member states.