Indianapolis, Indiana – An Indiana trademark attorney for Swag Merchandising, Inc. and  Devo Inc., both of California, sued in Hamilton Superior Court alleging that Your Fantasy Warehouse, Inc. d/b/a T.V. Store Online and Fred Hajjar, both of Commerce Township, Michigan, infringed Devo’s Trademarks, Registration Nos. 3161662 and 3167516, which have been registered by the U.S. Trademark Office. The case has been removed from Indiana state court to the Southern District of Indiana.
Devo Inc., both of California, sued in Hamilton Superior Court alleging that Your Fantasy Warehouse, Inc. d/b/a T.V. Store Online and Fred Hajjar, both of Commerce Township, Michigan, infringed Devo’s Trademarks, Registration Nos. 3161662 and 3167516, which have been registered by the U.S. Trademark Office. The case has been removed from Indiana state court to the Southern District of Indiana.
Swag claims that it owns the exclusive right to license the various trademarks, copyrights and individual and collective rights of publicity of the musical group Devo. The group is best known for the song “Whip It,” which hit number 14 on the Billboard chart in 1980. Swag indicates that it licenses the Devo intellectual property to third parties around the globe.
T.V. Store Online is in the business of manufacturing, marketing and distributing apparel and memorabilia featuring classic and current television programming, movies and/or music. T.V. Store Online and Hajjar have been accused of manufacturing, producing, marketing, advertising and/or retailing a product known as “Energy Dome Hats.” Plaintiffs assert that these Energy Dome Hats are commonly associated with Devo but have not been licensed by Plaintiffs to Defendants. Plaintiffs further claim that consumers coming into contact with Defendants’ product would “immediately recognize the same as being associated with, sponsored by and/or endorsed by” the ’80s group.
In the complaint, filed by an Indiana trademark attorney, Plaintiffs assert the following:
• I: Violation of 15 U.S.C. §1125(a) of the Lanham Act
• II: Trademark Infringement – 15 U.S.C. §1114 and Common Law
• III: Counterfeiting
• IV: Dilution – 15 U.S.C. §1125(c) and New York General Business Law §360-1
• V: Common Law Unfair Competition
• VI: Statutory Right of Publicity [NB: under Indiana law]
• VII: Right of Publicity Infringement Under California Civil Code §3344
• VIII: Common Law Right of Publicity
• IX: Conversion [NB: under Indiana law]
• X: Deception [NB: under Indiana law]
• XI: Indiana Crime Victims Act
Plaintiffs ask for an injunction; the surrender of infringing materials; damages, including treble damages; costs and fees. An Indiana intellectual property lawyer for Defendants removed the case to federal court, although he noted that the removal was not a concession that the Southern District of Indiana was the proper venue for the California Plaintiffs or the Michigan Defendants.
Practice Tip:
This is at least the third case filed by Theodore Minch about which we have blogged. In at least two prior cases, LeeWay Media Group, LLC v. Laurence Joachim et al. and Leon Isaac Kennedy v. GoDaddy et al., Mr. Minch has filed in an Indiana court despite none of the parties having any connection to Indiana.
It can be surmised that perhaps the choice of Indiana as a forum might have been driven by an attempt to increase damages. I.C. §§ 35-43-4-3 and 35-43-5-3(a)(6) are criminal statutes, claimed in the complaint in conjunction with an attempt to parlay the accusation into an award for damages, costs and attorneys’ fees. The Indiana Court of Appeals has discussed “theft” and “conversion” as they pertain to takings of intellectual property in several recent cases (see, for example, here and here) and has made it clear that criminal statutes often apply differently to an unlawful taking of intellectual property.
 Indiana Intellectual Property Law News
Indiana Intellectual Property Law News


 committed trademark infringement of “Tiki Tan”, Trademark Reg. No.
committed trademark infringement of “Tiki Tan”, Trademark Reg. No. Indiana (“Lilly”) filed a lawsuit in the
Indiana (“Lilly”) filed a lawsuit in the  unidentified John Does infringed the copyright of the motion picture “
unidentified John Does infringed the copyright of the motion picture “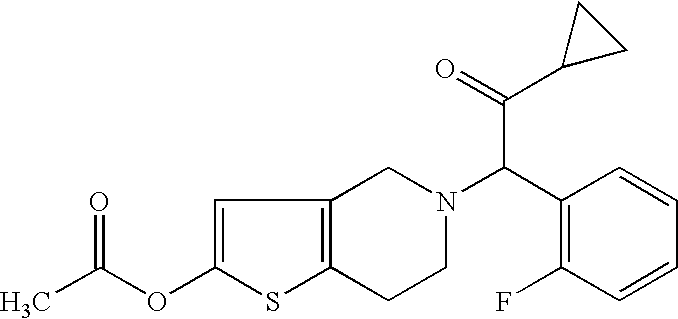 This is a civil action for patent infringement. It arises out of the filing by Defendant Par of an Abbreviated New Drug Application (“ANDA”) with the
This is a civil action for patent infringement. It arises out of the filing by Defendant Par of an Abbreviated New Drug Application (“ANDA”) with the  In this lawsuit, Plaintiff Contour Hardening contends that Defendant Vanair has violated, and continues to violate, inter alia, the patent laws of the United States, 35 U.S.C. §§271 and 281- 285, as well as the Federal Trademark Act by infringing Contour Hardening’s two patents, U.S. Patent Nos. 6,979,913 and 7,057,303 (collectively, the “Contour Patents”), and infringing Contour Hardening’s REAL POWER trademark by using Vanair’s allegedly similar ROAD POWER trademark.
In this lawsuit, Plaintiff Contour Hardening contends that Defendant Vanair has violated, and continues to violate, inter alia, the patent laws of the United States, 35 U.S.C. §§271 and 281- 285, as well as the Federal Trademark Act by infringing Contour Hardening’s two patents, U.S. Patent Nos. 6,979,913 and 7,057,303 (collectively, the “Contour Patents”), and infringing Contour Hardening’s REAL POWER trademark by using Vanair’s allegedly similar ROAD POWER trademark. intercepted and televised the Ultimate Fighting Championship 141:Brock Lesnar v. Alistar Overeem (“the Program”).
intercepted and televised the Ultimate Fighting Championship 141:Brock Lesnar v. Alistar Overeem (“the Program”). Indiana patent lawyer, has sued
Indiana patent lawyer, has sued  CleanTech sued
CleanTech sued IURTC is a not-for-profit corporation that fosters collaboration between
IURTC is a not-for-profit corporation that fosters collaboration between