Indiana Patent Litigation: Infringement of Patent for Furnace Cooling Pipes Alleged Against Foreign Defendants
Indianapolis, Indiana – Attorneys for Plaintiff, Amerifab, Inc. of Indianapolis, Indiana filed suit in the Southern District of Indiana alleging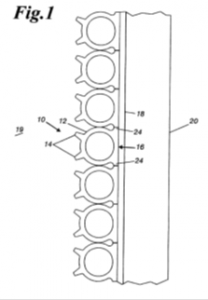 that Defendants, MELTER, S.A. a Mexican corporation; DE C.V., GERDAU AMERISTEEL CORPORATION, a Florida corporation; GERDAU S.A., a Brazilian corporation; and RAVAGNAN S.P.A., an Italian corporation infringed its rights in United States Patent No. 6,330,269 (“the ‘269 Registration”) for “Heat Exchange Pipe with Extruded Fins”. Plaintiff is seeking injunctive relief, judgment including statutory damages and attorneys’ fees.
that Defendants, MELTER, S.A. a Mexican corporation; DE C.V., GERDAU AMERISTEEL CORPORATION, a Florida corporation; GERDAU S.A., a Brazilian corporation; and RAVAGNAN S.P.A., an Italian corporation infringed its rights in United States Patent No. 6,330,269 (“the ‘269 Registration”) for “Heat Exchange Pipe with Extruded Fins”. Plaintiff is seeking injunctive relief, judgment including statutory damages and attorneys’ fees.
According to the complaint, Plaintiff Amerifab, Inc. manufactures equipment to be used in a variety of industrial machinery, including heat transfer equipment. Defendant Melter manufactures heat transfer equipment, pressure vessels, and markets in North America. Defendant Gerdau Ameristeel recycles scrap steel into products for the construction, industrial, agricultural, and automotive industries in North America. Gerdau Ameristeel is a wholly owned subsidiary of Defendant Gerdau, which operates subsidiaries primarily throughout South America. Defendant Ravagnan allegedly operates primarily out of Italy and South America, and specializes in design and construction of industrial plant and pressure vessels. Defendant Ravagnan is alleged the majority shareholder of Defendant Melter, and the two companies share facilities and employees.
 Indiana Intellectual Property Law News
Indiana Intellectual Property Law News