South Bend, Indiana – The Beachwaver Co. of Libertyville, Illinois filed a new lawsuit in the Northern District of Indiana alleging infringement of patents covering its rotating curling iron. Beachwaver seeks damages as well as injunctive and other relief under 35 U.S.C. 281, et seq.
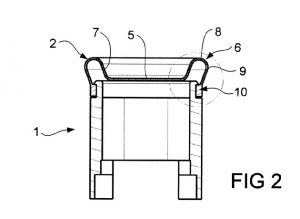
Defendants in this newest lawsuit are Xtava, LLC of Wilmington, Delaware, C&A IP Holdings, LLC of Dover, Delaware, and C+A Global of Edison, New Jersey. They are accused of infringing two patents: U.S. Patent Nos. 9,398,796 (“the ‘796 patent”) and 9,504,301 (“the ‘301 patent”). These patents, both titled “Hair Styling Device,” have been issued by the U.S. Patent and Trademark Office.
Plaintiff has filed two similar Indiana lawsuits asserting infringement in recent months, one in October and another in December. This most recent litigation, filed by Indiana patent lawyers for Beachwaver, lists two counts:
- Count 1: Infringement of the ‘796 Patent
- Count 2: Direct Infringement of the ‘301 Patent
Plaintiff seeks injunctive relief, costs, attorneys’ fees and damages, including treble damages.
 Indiana Intellectual Property Law News
Indiana Intellectual Property Law News