Indianapolis, Indiana – Patent attorneys for GS CleanTech Corporation of New York, New York (“CleanTech”), filed a patent infringement lawsuit in the Eastern District of California – Sacramento Division alleging that Pacific Ethanol Stockton LLC of Stockton, California infringed “METHOD OF PROCESSING ETHANOL BYPRODUCTS AND RELATED SUBSYSTEMS,” Patent No. 7,601,858 (the “‘858 patent”), which was issued by the U.S. Patent Office. The case was transferred to the Southern District of Indiana as part of Multidistrict Litigation No. 2181.
York (“CleanTech”), filed a patent infringement lawsuit in the Eastern District of California – Sacramento Division alleging that Pacific Ethanol Stockton LLC of Stockton, California infringed “METHOD OF PROCESSING ETHANOL BYPRODUCTS AND RELATED SUBSYSTEMS,” Patent No. 7,601,858 (the “‘858 patent”), which was issued by the U.S. Patent Office. The case was transferred to the Southern District of Indiana as part of Multidistrict Litigation No. 2181.
This litigation began with an assertion of patent infringement by CleanTech of the ‘858 patent, which was issued on October 13, 2009. CleanTech sued numerous Defendants alleging infringement of that patent shortly after its issuance. The Defendants accused of patent infringement in prior patent infringement litigation include: Big River Resources Galva, LLC; Big River Resources West Burlington, LLC; Cardinal Ethanol, LLC; ICM, Inc.; LincolnLand Agri-Energy, LLC; David J. Vander Griend; Iroquois Bio-Energy Co., LLC; Al-Corn Clean Fuel; Blue Flint Ethanol, LLC; ACE Ethanol, LLC; Lincolnway Energy, LLC; United Wisconsin Grain Producers, LLC; Bushmills Ethanol, Inc.; Chippewa Valley Ethanol Co.; Heartland Corn Products, Adkins Energy, LLC, Little Sioux Corn Processors, LLLP; Southwest Iowa Renewable Energy, LLC; Western New York Energy, LLC; Homeland Energy Solutions, LLC; Pacific Ethanol, Inc. [this entity is different from Pacific Ethanol Stockton LLC] and Guardian Energy, LLC. This Indiana patent litigation is being overseen in the Southern District of Indiana pursuant to the provisions for Multidistrict Litigation (“MDL”).
Since September 29, 2011, when the court overseeing the MDL issued its order on claim construction with respect to the disputed claims of the ‘858 patent, patentees CleanTech and its parent GreenShift Corp. have asserted patent infringement of three additional patents in the ‘858 family – U.S. Patent Nos. 8,008,516; 8,008,517 and 8,283,484 – against some of the allegedly infringing Defendants.
In this current patent infringement lawsuit, initiated by California patent lawyers in the Eastern District of California, subsidiary CleanTech is the sole Plaintiff. Patent attorneys for CleanTech assert only one count in the complaint: infringement of the ‘858 patent. CleanTech asks the court for preliminary and permanent injunctions prohibiting further infringement of the ‘858 patent; an award of damages adequate to compensate CleanTech for the infringement that has occurred, but in no event less than a reasonable royalty for the use made of the inventions of the ‘858 patent as provided in 35 U.S.C. § 284, together with prejudgment interest from the date the infringement began; and an award to CleanTech of all remedies available under 35 U.S.C. §§ 284 and 285.
Practice Tip: Previous articles about this litigation have posted many times by Indiana Intellectual Property Law News including:
CleanTech Adds Three Additional Defendants to Multidistrict Litigation
CleanTech Adds Western New York Energy as a Defendant in Patent Infringement Litigation
CleanTech’s Multidistrict Litigation Adds Southwest Iowa Renewable Energy as a Defendant
CleanTech Sues Aemetis for Infringing Patented Corn Oil Extraction Method
Southern District Allows CleanTech to Amend Complaint to Add Patent to Ethanol Patent Litigation
GS Cleantech Files Patent Infringement Lawsuit Against Flottweg in Indiana
Multiple Patent Infringement Suits Brought by Greenshift Consolidated in Indiana
GS CleanTech Sues Iroquois Bio-Energy for Patent Infringement
Cardinal Ethanol Sued by GS Cleantech for Patent Infringement
Little Sioux Added as a Defendant in CleanTech’s Multidistrict Litigation
 Indiana Intellectual Property Law News
Indiana Intellectual Property Law News


 filed a lawsuit in the
filed a lawsuit in the  Orthopaedics, Inc.
Orthopaedics, Inc. Illinois (also known as “John Deere”) infringed the patented “Combine Header Height Control“,
Illinois (also known as “John Deere”) infringed the patented “Combine Header Height Control“,  Aggregation,”
Aggregation,”  States Supreme Court
States Supreme Court Wauwatosa, Wisconsin (“Ruehl”) and PC Ruehl Engineering, Inc. of Wisconsin (“PC Ruehl”) filed patent infringement litigation in the
Wauwatosa, Wisconsin (“Ruehl”) and PC Ruehl Engineering, Inc. of Wisconsin (“PC Ruehl”) filed patent infringement litigation in the 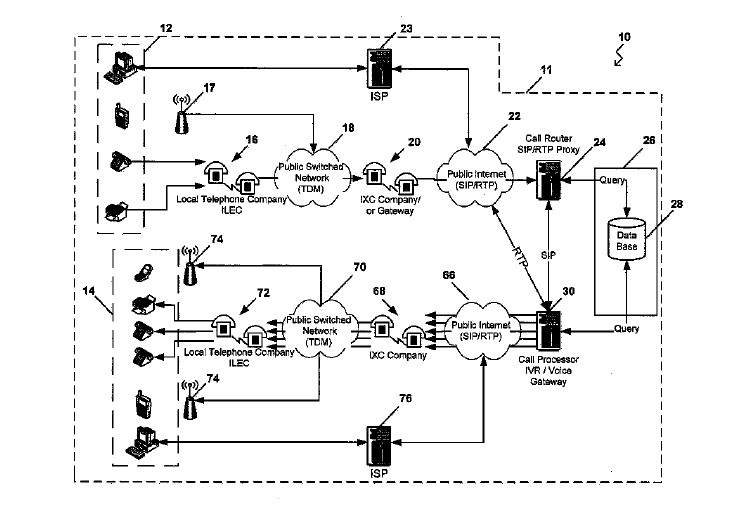 At issue in this patent litigation are the respective intellectual property rights of One Number and Google in single-phone-number telephone services. Such services allow a phone call made to one number to be transferred to multiple other phone numbers.
At issue in this patent litigation are the respective intellectual property rights of One Number and Google in single-phone-number telephone services. Such services allow a phone call made to one number to be transferred to multiple other phone numbers. Texas and
Texas and