Indianapolis, IN – The Southern District of Indiana has issued a claim construction order in a patent infringement lawsuit. Cook Incorporated, of Bloomington, Indiana, had filed a patent infringement lawsuit in the Southern District of Indiana against Endologix, Inc., of Irvine, California. The patents at issue are owned by Cook and are patent nos. 5,035,706, PERCUTANEOUS STENT AND METHOD FOR RETRIEVAL THEREOF and 5,755,777, 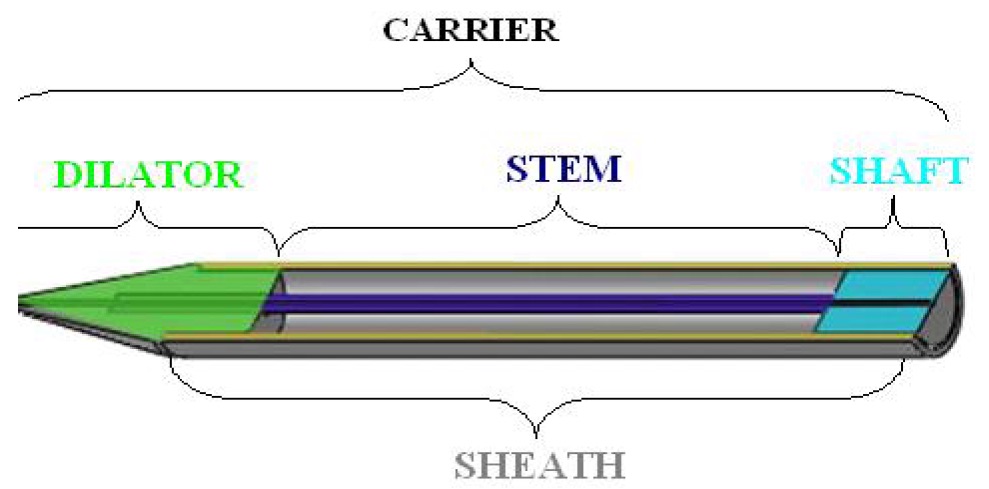 EXPANDABLE TRANSLUMINAL GRAFT PROSTHESIS FOR REPAIR OF ANEURYSM, which have been issued by the US Patent Office.
EXPANDABLE TRANSLUMINAL GRAFT PROSTHESIS FOR REPAIR OF ANEURYSM, which have been issued by the US Patent Office.
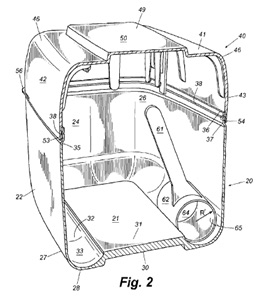
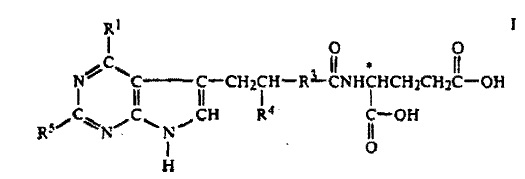
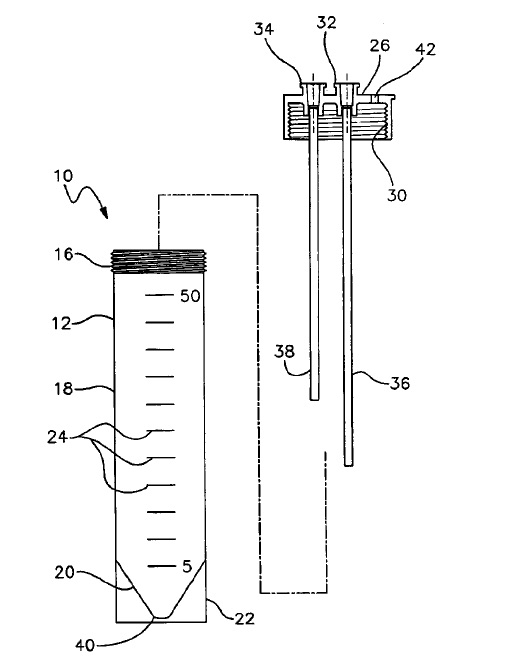
The patents at issue are medical devices that are used to treat abdominal aortic aneurysms, which are caused by the weakening of the aorta. The patented technologies are stents that strengthen the walls of the aorta and delivery systems. Cook alleges that Endologix’s Powerlink product infringes its patents. On April 15, 2011, the court held a Markman hearing to determine whether the patents cover the alleged infringer’s product. The parties disputed whether certain features of Endologix product were covered by Cook’s patents and the meaning of specific terms in the patent’s claims. Judge Pratt’s order set out the meaning of over thirty the disputed claim terms.
According to Yahoo Finance, Endologix has interpreted the rulings to be favorable.
Practice Tip: As the court described in its opinion, “[i]n order to win a patent infringement suit, a plaintiff must establish that the patent claim “covers the alleged infringer’s product or process.” Markman, 517 U.S. at 374 (citation and internal quotations omitted). Consequently, the first step in an infringement analysis involves determining the meaning and the scope of the words of the patent’s claims.” While resolution of disputed claim terms, such as what occurred in this opinion, does not resolve the controversy entirely, it is a significant step.
 Indiana Intellectual Property Law News
Indiana Intellectual Property Law News


 Communication network for a hospital bed,
Communication network for a hospital bed,