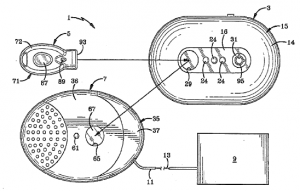
Indianapolis, Indiana – Attorneys for Plaintiff, Tyler Research Corporation (“TRC”), a corporation organized under the laws of Pennsylvania with its principal place of business in Alberta, Canada, filed suit in the Northern District of Indiana alleging that Defendants, Envacon, Inc., a Canadian corporation with a facility in Lafayette, Indiana, Keirnan Bozman, an individual residing in Alberta, Canada, and JKKB Holdings Corporation of Alberta, Canada, infringed its rights in United States Patent No. 6,273,053 (the “‘053 Patent”). Plaintiff is seeking a permanent injunction, damages, costs, expenses, both prejudgment and post-judgment interest, and any other relief the Court may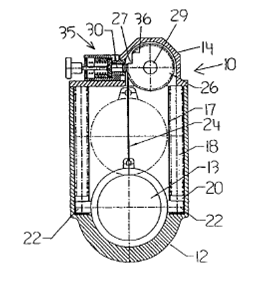 deem just and proper.
deem just and proper.
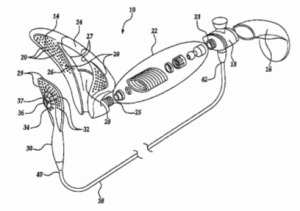
TRC, Bozman, and non-party, Joseph Krepela, entered into an exclusive licensing agreement granting TRC the exclusive right to manufacture Bozman and Krepela’s innovative engine shut off valves (the “Shut Off Valves”) on November 11, 1999. For consideration under the licensing agreement, TRC assisted with the design and completed development of the Shut Off Valves and provided employment to Bozman and Krepela. The licensing agreement did not limit TRC’s rights geographically and TRC’s exclusive right was to be in place so long as any patent rights of Bozman and Krepela existed in reference to the Shut Off Valves.
Bozman and Krepela were to put forth their best efforts in obtaining patent protection in Canada and the United States for the Shut Off Valves. Just short of four months after the exclusive licensing agreement with TRC was signed, Bozman and Krepela assigned their rights to the Shut Off Valves and the exclusive licensing agreement to JKKB. The very next day, JKKB filed for patent protection in the United States and Canada for the Shut Off Valves. The ‘053 Patent issued on August 14, 2001, with TRC holding the exclusive license to manufacture the Shut Off Valves.
Plaintiff exclusively manufactured the Shut Off Valves from 2001 until 2004, when it decided to outsource some of the manufacturing steps to Envacon, pursuant to the exclusive licensing agreement. Envacon, even before they began manufacturing some parts of the Shut Off Valves, had marketed and sold the completed Shut Off Valves for TRC and paid TRC’s parent corporation for TRC’s manufacturing services. TRC and Envacon operated out of the same premises from November 23, 1999 until April 16, 2011 and as such, TRC was able to maintain oversight, direction, and control over Envacon’s actions and involvement in manufacturing the Shut Off Valves.
Without giving prior notice, Envacon abandoned the space it shared with TRC on April 16, 2011. As TRC could no longer provide the necessary control and oversight over the manufacturing after Envacon left, Envacon was no longer authorized to manufacture the Shut Off Valves. TRC claims that Envacon, Bozman, and JKKB have all participated in the manufacturing of Shut Off Valves that infringe at least Claims 4, 14, and 15 of the ‘053 Patent or have conspired to deprive TRC of its rights under the exclusive licensing agreement. Plaintiff is claiming patent infringement against Envacon and Bozman, civil conspiracy against all Defendants, tortious interference against Envacon and Bozman, and civil conspiracy for tortious interference with a contractual relationship against Bozman and Envacon.
TRC of its rights under the exclusive licensing agreement. Plaintiff is claiming patent infringement against Envacon and Bozman, civil conspiracy against all Defendants, tortious interference against Envacon and Bozman, and civil conspiracy for tortious interference with a contractual relationship against Bozman and Envacon.
Continue reading
 Indiana Intellectual Property Law News
Indiana Intellectual Property Law News