Wilmington, DE – Indianapolis-based Eli Lilly & Company has won patent infringement protection of the drug Lilly’s drug ALIMTA drug, which is a chemotherapy drug used to treat mesothelioma and other lung cancers. Lilly co-owns the patent with Princeton University. The lawsuit arose from an Abbreviated New Drug Application filed with the Food and Drug Administration that had been filed by Teva Parenteral Medicines of Israel, alleging APP Pharmaceuticals LLC of Schaumburg, Illinois, and Barr Laboratories of Montvale, New Jersey. Their ANDA sought approval to sell generic versions of ALIMTA prior to the expiration of the patent.
As soon as the ADNA was filed, Lilly’s patent attorneys filed a patent infringement lawsuit in the District Court of Delaware. The defendants had claimed that the ALIMTA patent, patent no. 5,344,932, N-(pyrrolo(2,3-d)pyrimidin-3-ylacyl)-glutamic acid derivatives,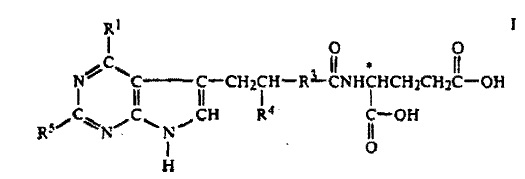 which has been issued by the US Patent Office, was invalid “under the doctrine of obviousness-type double patenting because the claimed invention is an obvious modification of inventions claimed in commonly-owned U.S. Patent Nos. 5,028,608 (“the ‘608 patent”) and 5,248,775 (“the ‘775 patent) in light of the relevant prior art.” The court held a five day bench trial in November 2010.
which has been issued by the US Patent Office, was invalid “under the doctrine of obviousness-type double patenting because the claimed invention is an obvious modification of inventions claimed in commonly-owned U.S. Patent Nos. 5,028,608 (“the ‘608 patent”) and 5,248,775 (“the ‘775 patent) in light of the relevant prior art.” The court held a five day bench trial in November 2010.
The Chief Judge Gregory Sleet wrote for the court in upholding the validity of the patent. “The court concludes that the examples found in the ‘775 patent specification do not support a finding of invalidity for obviousness-type double patenting because this case does not present a situation in which separate patents are sought for a claim to a compound and a claim to using that compound for the disclosed utility of the original compound.”
According to WISHTV.COM, Alimta was Lilly’s third best selling drug and had sales of $1.64 billion in first three quarters of 2010. The Alimta patent has been the subject of much litigation. On July 15, 2011, Lilly filed a patent infringement lawsuit regarding the same patent and against APP Pharmaceuticals, also a defendant in this case. Indiana Intellectual Law News blogged on the case here.
Practice Tip: As the court explained in this case: The doctrine of obviousness-type double patenting prevents a patentee from extending the term of exclusivity for a single invention by obtaining additional patents with only slight variations from the original invention. See Takeda Pharm. Co., Ltd v. Doll, 561 F.3d 1372, 1375 (Fed. Cir. 2009). Obviousness-type double patenting “prohibits claims in a later patent that are not patentably distinct from claims in a commonly owned earlier patent.” Sun Pharm. Indus., Ltd v. Eli Lilly & Co., 611 F.3d 1381, 1384 (Fed. Cir. 2010).”Decision
 Indiana Intellectual Property Law News
Indiana Intellectual Property Law News

