South Bend, Indiana – Indiana patent attorneys for Cor-A-Vent Inc. of Mishawaka, Indiana 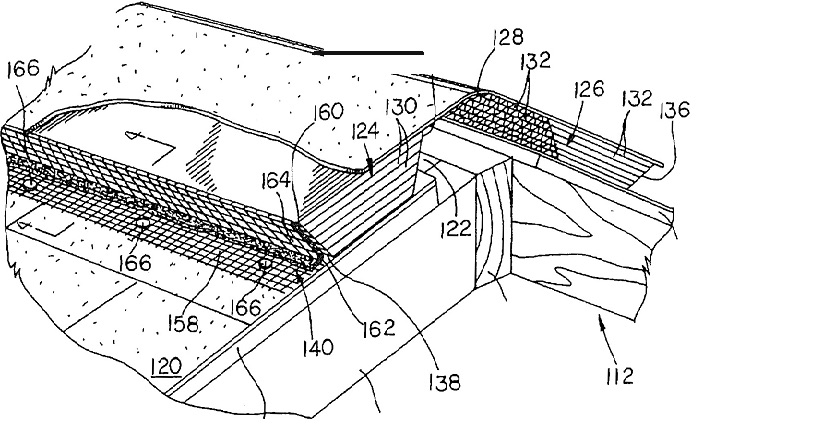 filed a lawsuit in the Northern District of Indiana alleging that Designer Cabinets Inc. d/b/a DCI Products of Clifton Heights, Pennsylvania (“DCI”) infringed the patented “Moisture Resistant Roof Vent,” Patent No. 5,704,834, which has been issued by the U.S. Patent Office.
filed a lawsuit in the Northern District of Indiana alleging that Designer Cabinets Inc. d/b/a DCI Products of Clifton Heights, Pennsylvania (“DCI”) infringed the patented “Moisture Resistant Roof Vent,” Patent No. 5,704,834, which has been issued by the U.S. Patent Office.
Cor-A-Vent is a designer and manufacturer of roof vent and other venting products. In 1998, United States Patent No. 5,704,834 (the “‘834 Patent”) was issued for a moisture-resistant roof vent. Cor-A-Vent asserts that it owns this patent.
DCI is accused of infringing the ‘834 Patent – either directly, by inducement, and/or contributorily – by making, using, selling, offering for sale, importing or supplying infringing roof vent products, including DCI’s SmartRidge® II product, in violation of 35 U.S.C. § 271 et seq. Cor-A-Vent contends that DCI has profited, and Cor-A-Vent has suffered damages, as a result of this alleged patent infringement.
Cor-A-Vent also asserts that DCI has been aware of the ‘834 Patent since 2010 or earlier. As a result, Cor-A-Vent contends that the patent infringement committed by DCI has been willful. Further, Cor-A-Vent contends that this is an exceptional case, which would support an award of reasonable attorneys’ fees pursuant to 35 U.S.C. § 285.
In its complaint, filed by Indiana patent attorneys in Indiana federal court, Cor-A-Vent lists a single count: Infringement of United States Patent No. 5,704,834. It requests the following:
• A judgment that DCI has infringed the ‘834 Patent in violation of 35 U.S.C. § 271;
• Preliminary and permanent injunctive relief prohibiting DCI and its agents from infringing the ‘834 Patent pursuant to 35 U.S.C. § 283;
• An award to Cor-A-Vent of its damages for patent infringement,
• An award of pre-judgment and post-judgment interest and costs against DCI pursuant to 35 U.S.C. § 284;
• A judgment that DCI’s infringement of the ‘834 Patent has been deliberate and willful;
• A judgment that DCI’s infringement of the ‘834 Patent has been exceptional under 35 U.S.C. § 285;
• Treble damage under 35 U.S.C. § 284; and
• An award to Cor-A-Vent of its reasonable attorneys’ fees under 35 U.S.C. § 285.
Practice Tip:
A court may award increased damages for willful infringement. These punitive damages, up to and including a trebling of damages, are appropriate when an infringer has acted in wanton disregard of the patentee’s intellectual property rights. In determining whether the infringing behavior supports increased damages, the court will consider the “totality of the circumstances.”
Potential exposure for increased damages may be reduced by seeking – and acting on – timely advice from a competent patent lawyer. In contrast, the failure to seek and heed such advice may increase the chance of a finding of willfulness.




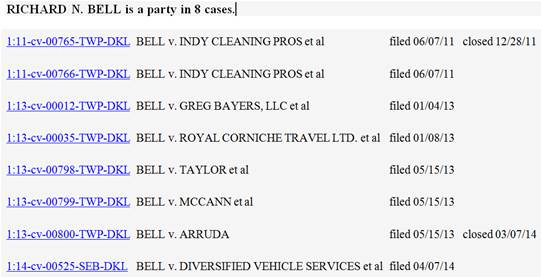 Defendants are: Diversified Vehicle Services of Marion County, Indiana; Cameron Taylor and Taylor Computer Solutions of Indianapolis, Indiana; Rhonda Williams of Indianapolis, Indiana; Forensic Solutions, Inc. of Waterford, New York; Heath Garrett of Nashville, Tennessee; CREstacom, Inc. of Fishers, Indiana; American Traveler Service Corp LLC, location unknown;
Defendants are: Diversified Vehicle Services of Marion County, Indiana; Cameron Taylor and Taylor Computer Solutions of Indianapolis, Indiana; Rhonda Williams of Indianapolis, Indiana; Forensic Solutions, Inc. of Waterford, New York; Heath Garrett of Nashville, Tennessee; CREstacom, Inc. of Fishers, Indiana; American Traveler Service Corp LLC, location unknown; “IP Jurisprudence in the New Technological Epoch: The Judiciary’s Role in the Age of Biotechnology and Digital Media.” The program will run from 9 a.m. to 5 p.m. and will provide 6.5 hours of continuing legal education.
“IP Jurisprudence in the New Technological Epoch: The Judiciary’s Role in the Age of Biotechnology and Digital Media.” The program will run from 9 a.m. to 5 p.m. and will provide 6.5 hours of continuing legal education. individuals infringed trademarks for “ORDER INN”, Registration Nos.
individuals infringed trademarks for “ORDER INN”, Registration Nos.  Indianapolis, Indiana filed a lawsuit in the
Indianapolis, Indiana filed a lawsuit in the  “STRATOTONE” (the “Stratotone mark”),
“STRATOTONE” (the “Stratotone mark”),  Washington – The U.S. Department of Commerce’s
Washington – The U.S. Department of Commerce’s  Orthopaedics, Inc.
Orthopaedics, Inc.