Glitch Productions Pty Ltd, an Australian animation studio behind hit web series like The Amazing Digital Circus, has filed a lawsuit against various individuals and businesses operating e-commerce stores under various aliases. These stores are accused of selling counterfeit merchandise featuring Glitch’s registered trademarks without permission.
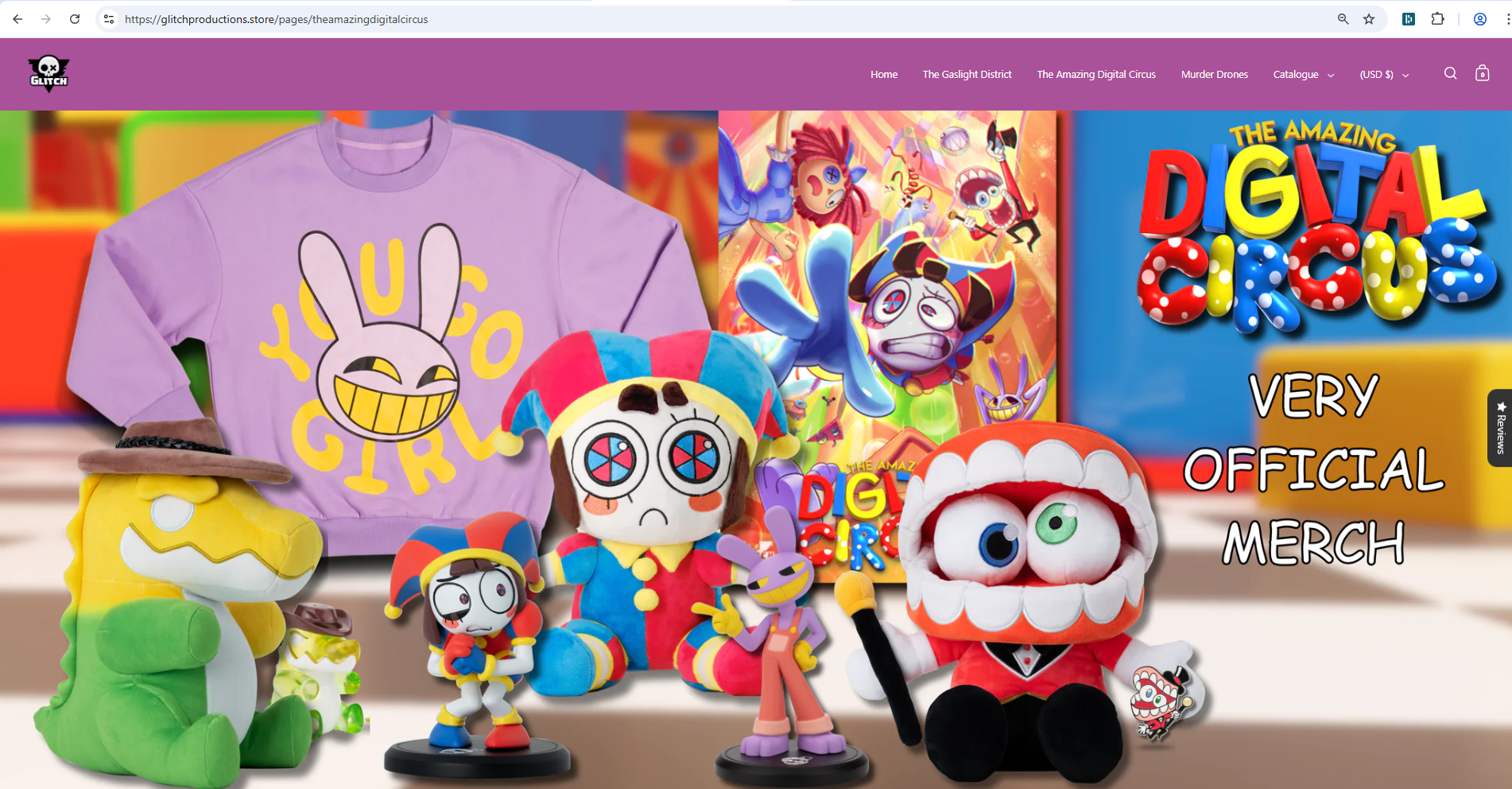 Founded in 2017, Glitch is known for its distinctive 3D animation style and has built a global fanbase with over 8.5 million YouTube subscribers. Its show The Amazing Digital Circus, created by Gooseworx, became a viral success after its October 2023 debut, amassing hundreds of millions of views and expanding to Netflix in 2024. Glitch sells official merchandise—such as clothing, figures, and posters—through its online store, with trademarks recognized in the U.S. and internationally.
Founded in 2017, Glitch is known for its distinctive 3D animation style and has built a global fanbase with over 8.5 million YouTube subscribers. Its show The Amazing Digital Circus, created by Gooseworx, became a viral success after its October 2023 debut, amassing hundreds of millions of views and expanding to Netflix in 2024. Glitch sells official merchandise—such as clothing, figures, and posters—through its online store, with trademarks recognized in the U.S. and internationally.
The complaint alleges that the defendants target U.S. consumers, including in Indiana, by running interactive storefronts that accept U.S. dollars, ship domestically, and present counterfeit items as official products. Glitch claims the defendants intentionally copy its branding to mislead buyers and weaken its trademark value. The company states it has not authorized these sellers and that their actions violate its intellectual property rights.